Liquid-liquid phase separation
Liquid-liquid phase separation (LLPS) occurs when molecules spontaneously segregate into distinct liquid phases. A common example is the vinaigrette, where oil and water separate into distinct layers. In biological cells, LLPS drives the formation of membraneless organelles, also known as biomolecular condensates—liquid droplets that create compartments without the need for membranes.
These condensates play crucial roles in cellular organization and act as hubs for biochemical reactions. Their malfunction can lead to diseases such as cancer and neurodegenerative disorders.

Some key questions that we seek to address:
- How do nonequilibrium activities in the cell like reactions and active forces interplay with condensates?
- How do condensates interact with and organize the complex structures (e.g. chromatin, membrane, and filaments) within cells?
- How do condensates transition between liquid-like and gel-like states?
Publications:
- Condensate-driven interfacial forces reposition DNA loci and measure chromatin viscoelasticity, Amy R. Strom, Yoonji Kim, Hongbo Zhao, Natalia Orlovsky, Yi-Che Chang, Andrej Košmrlj, Cornelis Storm, Clifford P. Brangwynne, Cell (2024) Read more
- Mapping and engineering RNA-controlled architecture of the multiphase nucleolus
Sofia A. Quinodoz, Lifei Jiang, Aya Abu-Alfa, Troy J. Comi, Hongbo Zhao, Qiwei Yu, Lennard W. Wiesner, Jordy F. Botello, Anita Đonlić, E. Soehalim, Christiane Zorbas, L. Wacheul, Andrej Košmrlj, Denis L.J. Lafontaine, Sebastian Klinge, Clifford P. Brangwynne Read more
Motility-induced phase separation
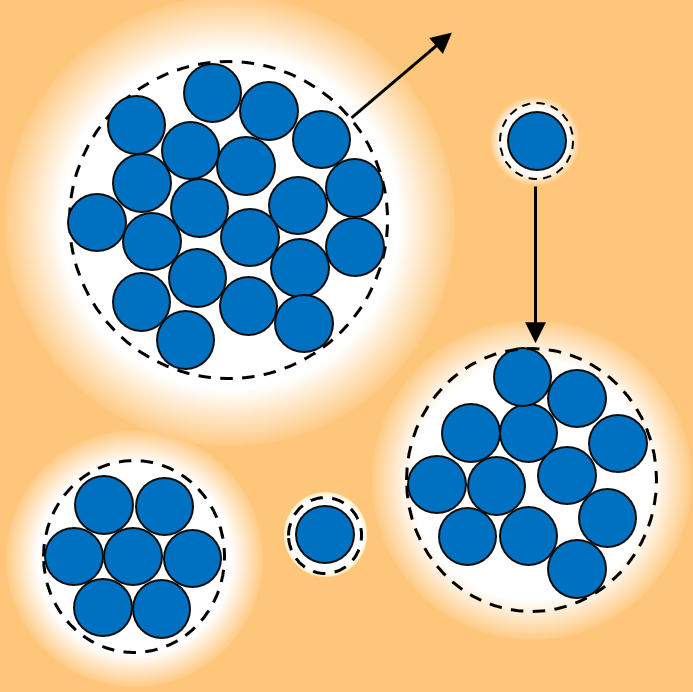
Many microbial organisms, such as bacteria, are motile, enabling them to move and navigate their environments. Motility-Induced Phase Separation (MIPS) describes a remarkable phenomenon where actively moving particles, such as motile cells or synthetic active matter, spontaneously cluster into distinct phases without any attractive interactions. This behavior sheds light on how microbial communities and synthetic active matter organize and function.
We are particularly interested in exploring the interplay between MIPS and the complex environments inhabited by microbiomes as well as other modes of motion, such as chemotaxis, which enables bacteria to sense and move toward nutrient-rich areas.
Publications:
- Chemotactic motility-induced phase separation, Hongbo Zhao, Andrej Košmrlj, Sujit Datta, Physical Review Letters (2023) Read more
Phase transition in nonequilibrium systems
Phase transition and chemical reactions are both classical subjects in chemical physics, yet their interplay remains less well understood. We find chemical reactions can drastically alter phase behavior. For example, reactions can suppress phase separation in systems that would naturally phase-separate under thermodynamic conditions, or induce phase separation in systems that otherwise remain mixed.
Nonequilibrium and chemically active systems are ubiquitous in our daily lives, from ATP-driven processes that power cellular functions to the electrochemical reactions that occur in the batteries of our cellphones. These studies not only advance nonequilibrium physics but also have practical implications for fields such as biology and energy technology.
Publications:
- Theory of chemically driven pattern formation in phase-separating liquids and solids Hongbo Zhao, Martin Z. Bazant Read more
- Fictitious Phase Separation in Li Layered Oxides Driven by Electro-Autocatalysis,(*: equally contributing) Jungjin Park*, Hongbo Zhao*, Stephen Dongmin Kang*, Kipil Lim, Chia-Chin Chen, Young-Sang Yu, Richard D. Braatz, David A. Shapiro, Jihyun Hong, Michael F. Toney, Martin Z. Bazant, and William C. Chueh Nature Materials (2021) Read more
- Population dynamics of driven autocatalytic reactive mixtures
Hongbo Zhao, Martin Z. Bazant Physical Review E (2019) Read more